Starlims is pleased to announce the release of Starlims SDMS (Scientific Data Management System) V12.2. In addition to being available as part of the Starlims integrated solution, it is now available as a standalone product to help clients achieve compliance with the data integrity expectations of the Food and Drug Administration (FDA) as well as other regulatory authorities.
Starlims SDMS V12.2 can work with an existing LIMS or without a LIMS, interfacing effectively with any LIMS (Laboratory Information Management System), CDS (Chromatography Data System), ELN (Electronic Laboratory Notebook), SAP (Systems Applications Products) and other lab systems through webservices without replacing them.
Starlims SDMS V12.2 can benefit three types of clients:
- Organizations purchasing the Integrated Starlims Laboratory Information Management Solution which includes SDMS
- Organizations with an existing LIMS lacking SDMS capabilities
- And organizations without a LIMS, seeking a Standalone SDMS solution to manage their data and better meet regulatory requirements
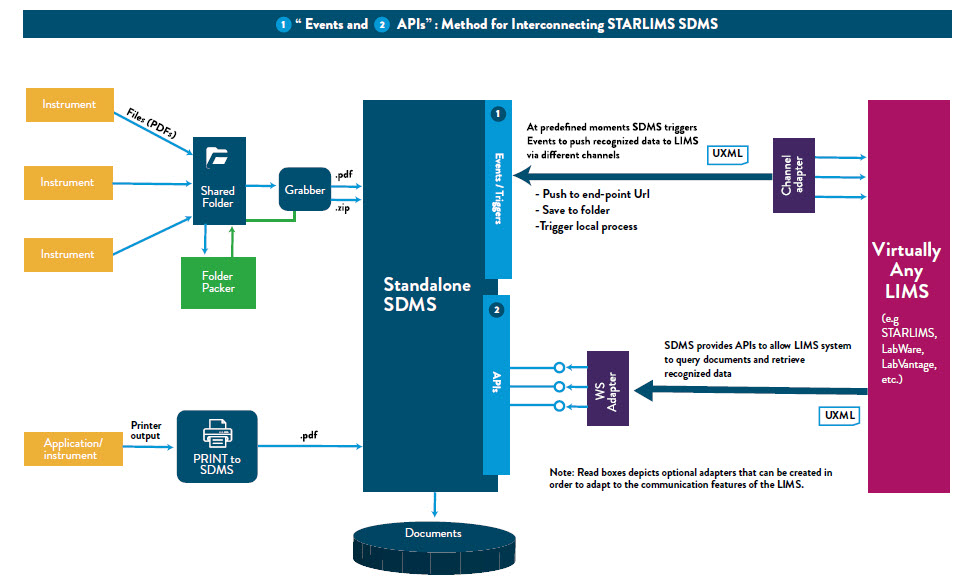
Starlims designed this solution specifically to help organizations comply with 21CFR Part 11 regulations; to ensure compliant record creation, audit trails, electronic signatures and data security and to offer the following benefits to clients:
- Identification and storage of equipment data files in native formats
- The ability to parse data, including extracted metadata
- No requirement of the original software to create the record
- Review of data before it is stored or sent to other applications
Starlims Standalone SDMS can work independently or with an integrated LIMS system to achieve data integrity by capturing data in real time, backing up data, protecting it from deterioration and loss, and maintaining the raw data as the original record. All these benefits will help clients maintain quality control, minimize errors and meet regulatory requirements.