Overcome Common Lab Challenges with These Six LIMS Best Practices
Imagine this: You’re managing a laboratory bustling with activity, generating mountains of data every day. Your team struggles with manual processes, siloed systems, and mounting pressure to achieve compliance with ever-changing regulations. The stakes are high—errors could lead to costly delays, regulatory fines, or even jeopardize your company’s reputation.
Here’s where the Laboratory Information Management System (LIMS) comes in. Created to transform your lab operations, LIMS support lab efficiency, productivity, and security while enabling compliance.
But adopting a LIMS is only the first step. To truly harness its power, you need to implement best practices that will unlock its full potential and address your most pressing pain points. Let’s explore six essential steps to help your organization maximize the value of your LIMS investment and overcome common laboratory challenges.
1. Keep Your Valuable IP Secure
In today’s interconnected world, data security is of utmost importance, especially when you are managing information that provides your company with a competitive advantage and enables you to secure your IP.
With a LIMS, you can implement security measures to protect your IP and sensitive information, such as:
- Regular password security upkeep: Enforce strong password policies and require periodic updates.
- Firmly defined user privileges: Assign roles and responsibilities to ensure only authorized personnel have access to critical data.
- Access controls: Use multi-factor authentication for secure access.
- Robust data backup and recovery mechanisms: Implement automated backups and disaster recovery plans.
- Proper disposal of digital files: Ensure obsolete files are securely deleted to prevent unauthorized access.
LIMS can also minimize the risk of data breaches by enabling encryption and advanced access control, safeguarding your IP and sensitive information from external and internal threats.
2. Configure or Customize… That Is the Question
There is some debate between in the world of software over what is better: Customization or Configuration.
Customized Software: Built specifically for an organization’s unique requirements. While this may sound ideal, it often leads to high costs, scalability issues, and challenges in maintaining the system. Over time, a heavily customized system may deviate from its original purpose and become overly complex.
Configurable Software: Offers flexibility to adapt the system to your needs without altering its core structure. Configurable LIMS solutions are simpler, faster, and more cost-effective to implement. They also allow you to align with industry best practices and stay current with software trends, leading to quicker ROI realization.
Our recommendation? Whenever possible, opt for a configurable LIMS that can evolve with your organization and industry requirements.
3. Select Your Deployment for Longterm Success
LIMS implementations are challenging enough without worrying about the right IT infrastructure. Unsure which hosting option suits your needs? Generally, it boils down to two different options—cloud deployment or on-premise deployment—with nuances to each. There are benefits to both options, such as:
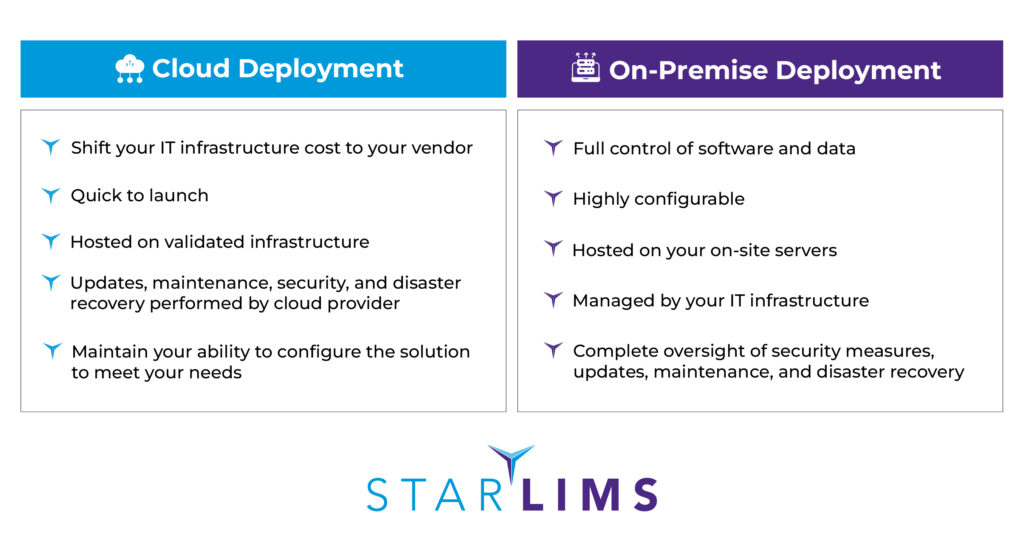
4. Simplify Compliance
Compliance is a significant challenge for laboratories, encompassing regulatory, customer, and internal standards. A robust LIMS can:
- Streamline regulatory compliance: Support adherence to industry standards, government regulations, and internal quality standards.
- Support customer compliance objectives: Tailor workflows and reports to meet specific client requirements.
- Facilitate internal compliance: Implement consistent protocols across teams and locations.
With features like automated documentation, real-time monitoring, and electronic audit trails, LIMS can simplify compliance processes, reduce errors, and ensure a higher standard of quality in your operations.
5. Unify Your Lab Processes with LIMS Consolidation
Operating across multiple, disparate LIMS systems can create inefficiencies, data silos, and resource drains. By consolidating to a single, unified LIMS, you can:
- Establish standardized processes for data handling, reporting, and compliance.
- Improve data integrity and accuracy by reducing manual errors.
- Gain a centralized view of lab operations, facilitating better decision-making and collaboration.
A unified LIMS provides a solid foundation for achieving streamlined workflows, secure data sharing, and improved operational efficiency across your organization.
6. Consider Building Out Your Lab Tech Ecosystem
LIMS is just one piece of the lab technology puzzle. To fully optimize your lab operations and stay ahead in today’s competitive landscape, consider building a comprehensive lab tech ecosystem.
For example, the STARLIMS R&D Quality Manufacturing Informatics Platform encompasses our Quality Manufacturing LIMS and:
- Labstep Electronic Lab Notebook: Built by scientists, for scientists to help you accelerate ideation and simplify experiment execution in R&D.
- Lab Execution System: Take your LIMS on the go. Enable your team to perform SOPs precisely while at the lab bench and in the field.
- Scientific Data Management Systems: Streamline lab and quality data management with a centralized repository and simplify testing equipment integration.
- Advanced Analytics 2.0: New and vastly improved! Make critical decisions faster with better data.
By integrating these tools, you can create a seamless ecosystem that supports your lab operations from ideation to final product delivery.
Read the Ultimate Guide to LIMS

If you enjoyed this blog, there’s more to explore! This article is just one chapter of the new STARLIMS ebook, The Ultimate Guide to LIMS for Manufacturers.
This definitive guide provides insights into selecting the best LIMS for your needs, implementing it effectively, and following best practices for ongoing success. Don’t miss out on the opportunity to master LIMS and take your lab operations to the next level!

